Andrade-Lab Research
Our lab is interested in understanding the biochemical and biophysical aspects ruling the function of PROTEINS. Our current main targets are AMMONIUM TRANSPORTERS (Amt) and AMMONIUM RECEPTORS (AmR), together with their physiological partner proteins. To understand how they function at molecular detail, we combine various techniques to obtain structural and functional clues.
AMMONIUM TRANSPORTERS
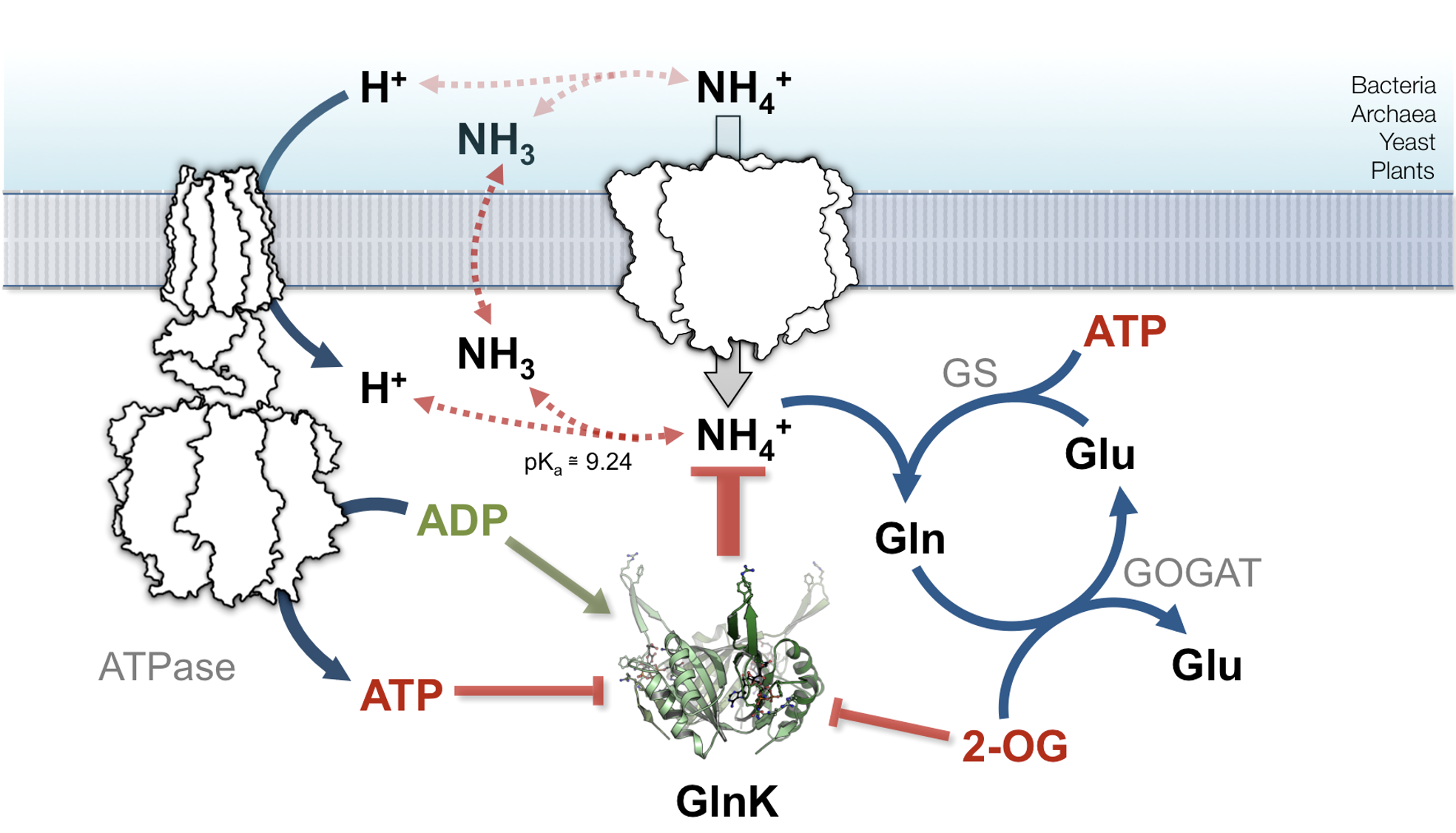
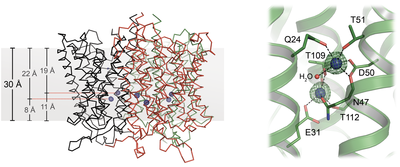
Ammonium transporters like the Archaeoglobus fulgidus Af-Amt1 protein (pubmed.ncbi.nlm.nih.gov/16214888/) are trimers of eleven transmembrane helices per monomer. Each monomer contains a highly selective translocation pore for ammonium (and methylammonium) cations. The core architecture of the protein and arrangement of residues along the pore are broadly conserved, up to the human Rhesus homologs.
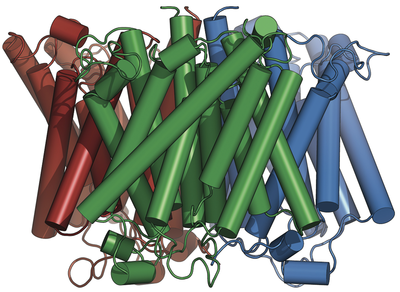
We did not consider these proteins to function as ammonia (NH3) gas channels in vivo (pubmed.ncbi.nlm.nih.gov/17710640/) and therefore, directed much of our efforts into establishing a reliable and direct assay to monitor their transport abilities.
Using solid-supported membrane (SSM) electrophysiology, we recorded electrogenic currents from proteoliposomes containing Amt proteins and proved that (also prokaryotic) Af-Amt1 and Af-Amt3 mediate the slow transport of NH4+ ( 30-300 ions.sec-1.trimer-1) across membranes (pubmed.ncbi.nlm.nih.gov/24958855/).
The detailed description of the transport process has been elusive, likely due to the fact that particular functional characteristics such as their extreme selectivity towards ammonium cation, is the product of a larger architectural feature and not just of a small set of amino acid residues. A deeper understanding of the transport mechanism is one of our main research goals.
AMMONIUM RECEPTORS
Ian 2018 we published the first example of an Amt that preserves all pore residues considered essential for ammonium selection and transport, but instead of transporting it, deviates two cations into a binding pocket within each monomer to function as a receptor instead (pubmed.ncbi.nlm.nih.gov/29323112/):
This "Candidatus Kuenenia stuttgartiensis" protein, Ks-Amt5, contains a fused cytoplasmic histidine kinase domain that acts as the transducer of the initial, external ammonium signal, to a yet unknown response regulator protein. The structural characterization of the protein is so far limited to its membrane Amt-like receptor module due to the high flexibility of the transducer domain. We expect that single-particle cryo-EM studies can improve this and help us provide a structural perspective of the functional modulation of these systems.
This protein is not unique and we are researching other ammonium receptors (AmRs), to help us validate features and comprehend a number of mechanistic aspects of Amt proteins as well.
PII PROTEINS (GlnK)
GlnK proteins belong to the PII family and occur in an operon, upstream or downstream amt genes. Typical PII proteins are cytoplasmic trimeric sensors of the energy and carbon/nitrogen cellular levels with binding of Mg-ATP, ADP or Mg-ATP:2-oxoglutarate triggering alterations in a protruding T-loop region that dictate their affinity to downstream partner proteins. Some PII proteins can additional suffer additional modulation by covalent modifications.
We are interested in characterizing the GlnK proteins from Archaeoglobus fulgidus, Af-GlnK1 (pubmed.ncbi.nlm.nih.gov/21301082/), Af-GlnK2 (pubmed.ncbi.nlm.nih.gov/20643148/) and Af-GlnK3 (pubmed.ncbi.nlm.nih.gov/22039461/) to understand the interaction with their respective Amt partner.
We observed differences in their ligand binding affinities and cooperative behavior. We also noted that Af-GlnK2 did not to recognize 2-oxoglutarate (2-OG) as a signal for Amt:GlnK complex dissociation. This was the first identified case of 2-OG insensitive PII protein (pubmed.ncbi.nlm.nih.gov/20643148/).